Tertahadral & Octahedral Voids, HCP & CCP
Concepts of Voids, HCP & CCP Arrangements
Density of unit cell-
Tetrahedral and Octahedral void-
Tetrahedral Void-
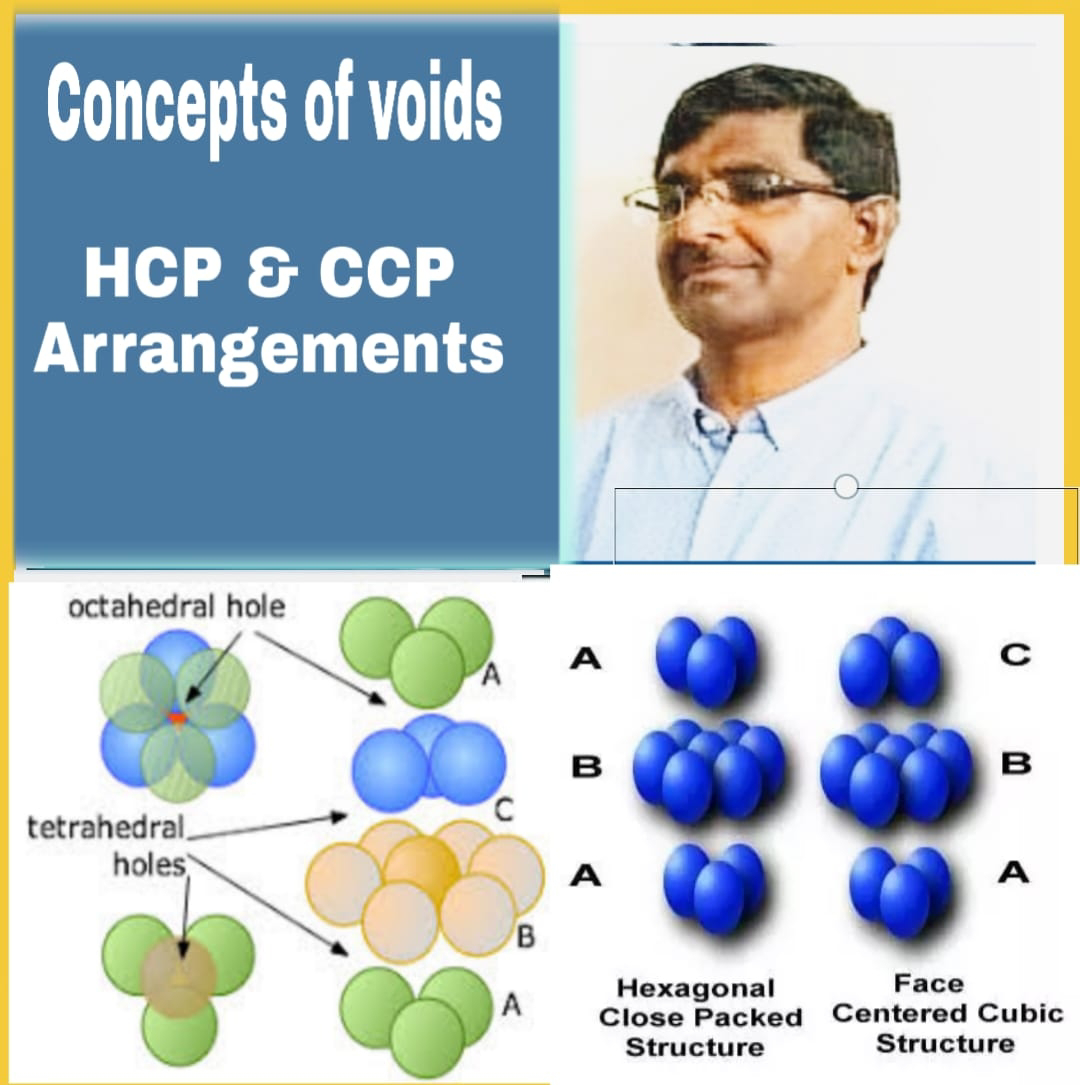
A simple triangular void in a crystal which is surrounded by four spheres is called tetrahedral void.
A tetrahedral void is developed when triangular voids have contact with one sphere either in the upper layer or in the lower layer. Tetrahedral void means the tetrahedral arrangement of spheres.
.
Characteristics of tetrahedral void-
(i) Ratio of radii of void and constituent particle is constant.
r/R = 0.225
(ii) Co-ordination number of particle present in the void is 4
(iii) Each constituent particle makes two tetrahedron with three spheres above and three spheres below
(iv) No. of tetrahedral voids
= 2 ×No. of atoms
= 2× octahedral voids
Relationship B/W radius of void (r) and radius of atom(R)
The double triangular void which is surrounded by six spheres is called octahedral void. In this void out of the six spheres , four are placed in the same plane touching each other, one sphere is placed from above and the other from below the plane of these spheres.
Characteristics of octahedral void-
(i) Ratio of radii of void and constituent particle is constant.
=0.414
(ii) Coordination of particle present in the void is 6
(iii) Size of octahedral void is greater than tetrahedral void
(iv) No. of octahedral void
= No. of atoms
= 1/2 × Tetrahedral void
Relationship B/W radius of void (r) and radius of atom(R)
in this structure atoms are arranged at the corners and at the centres of all the six faces of a cube.
Cubic close packing is more efficient than hcp .The ccp structure has following features-
(i) Each atom is surrounded by 12 others and is said to have coordination no. 12.
(ii) It has 3 fold axis of symmetry.
(iii) This type of packing give ABC, ABC,ABC………….layers type of arrangement
(iv) CCP arrangement of atoms occupy 74 % of available space
(v) It has four sets of parallel close packed layers, hence the chance for slipping of one layer over the other are more
Example- Al , Cr , Co , Cu , Ag , Fe et.
Hexagonal closed packing (HCP)- In this arrangement atoms are located at the corners and centre of two parallel hexagons ,three more atoms are placed in a parallel plane midway between these two planes.
HCP arrangement has following features-
(i) Each atom is surrounded by 12 others and is said to have coordination no. 12.
(ii) It has 6 fold axis of symmetry.
(iii)This type of packing give AB, AB, AB……..layers type of arrangement
(iv) HCP arrangement of atoms occupies 74 % of the available space.
(v) It has only one set of parallel close packed layers .Hence chance for slipping of one layer over the other is very less.
(v) Fraction of volume available in hcp is = =0.74
Example- Be, Cd, Li ,Mg , Zn etc.