Crystal System & Bravais Lattices
Crystal system and Bravais lattices-
Crystal system and Bravais lattices-
Based upon the dimensions of the unit cells (i.e. length a, b, and c and the angles (α , β and γ ) there are seven types of unit cells. These are also called crystal systems are crystal habits because any crystalline solid must belong to any one of the unit cells.
“At first sight there seems to be infinite number of shapes of crystals. However careful examination of several thousand crystals of various substances has revealed that only seven possible symmetries are possible. The seven different combinations of symmetry elements are called crystal systems.”
These crystal systems differ in the length of the unit cell edges (a, b and c) and angles ( α,
β and γ ) between the unit cell The arrangement of particles in the study of crystal was first given by German Scientist M. Frankenheim in 1844.
Classification on the basis of geometrical consideration-
In 1848 A. Bravais studied geometrical arrangement of crystals on the basis of three fundamental rules of crystallography According to Bravais there are 14 space lattices are possible in several thousand crystals. In other words lattice points are arranged in space by 14 specific combinations.
A.Bravais is also called as father of chemical crystallography.
“ The fourteen lattices corresponding to seven crystal systems are known as Bravais lattices.”
Seven types of unit cells or crystal system
According to a, b and c and α ,β and γ
there may be 230 possible shapes which are grouped in to 32 classes, these 32 classes are again grouped into 14 Bravais lattices, which collectively form 7 crystal system
Co-ordination Number-
The number of oppositely charged ions surrounding a particular ion in the ionic crystal is called its co-ordination number or Gold-Smidt number. The concept was given by Goldsmidt and Alfred Werner.
It is the characteristics of a space lattice and its value can be calculated by observing the crystal lattice.
The value of co-ordination number of simple cubic lattice (SC) is 6
The value of coordination no of BCC is 8
The value of co-ordination number of face centred cubic (FCC) is 12.
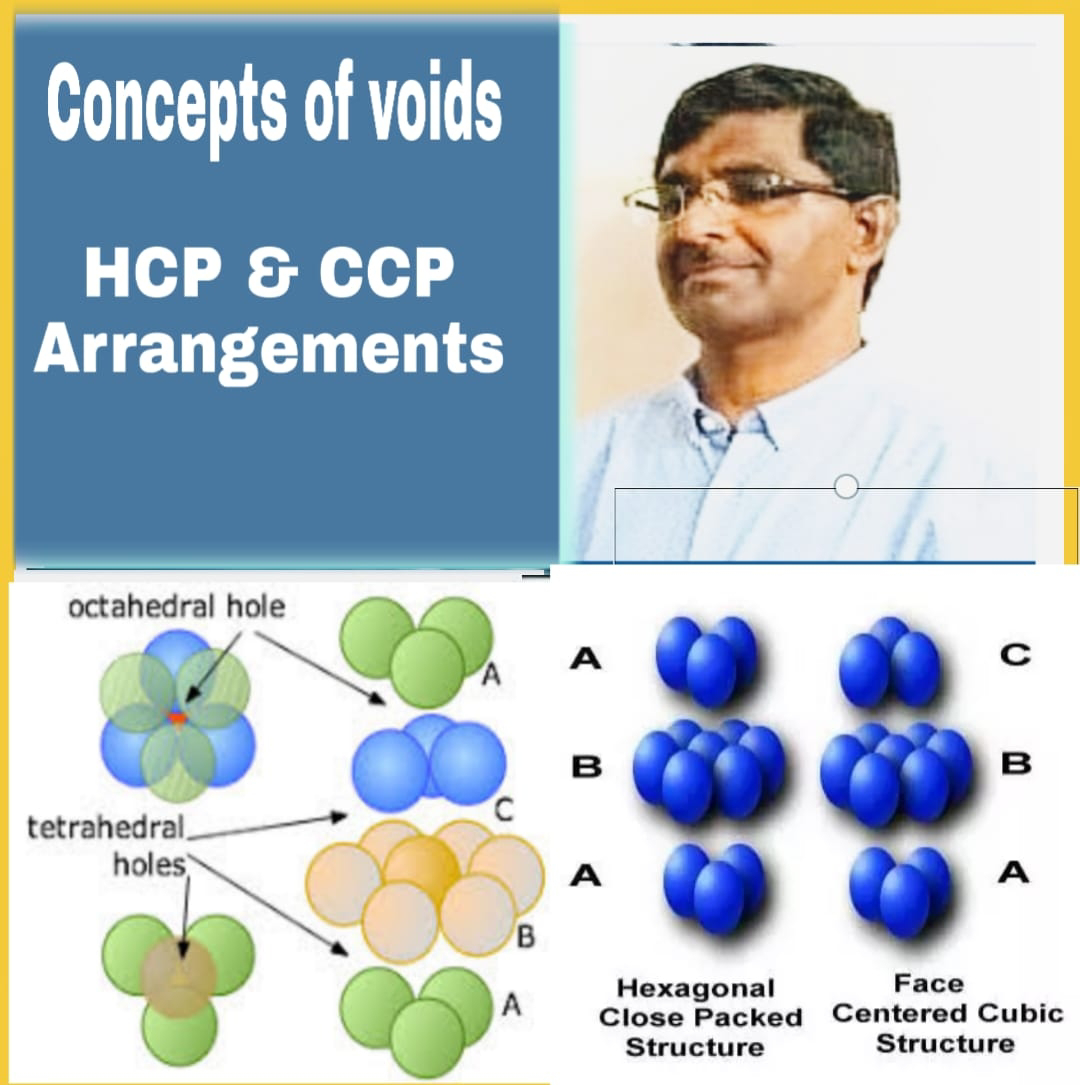
The value of co-ordination number of hexagonal closed packing (HCP) and cubic closed packing (CCP) is 12.