Crystal Defects or Crystal Imperfections
Crystal Defects or Imperfections-
Any departure from perfectly ordered arrangement of atoms in crystal is called crystal defects or imperfection.
Schottky, International and Anti structure are three basic types of point secrets. In additional three hybrid types of de devta can aise from combination of two of basic types. Frankel effect is more common in tonic solid.
It is not possible to explain many properties of solids such as electrical conductivity and mechanical strength on the basis of structure alone . Hence defects not only modify the properties but also impart some other properties of solids.
Point Defect- The defect caused by missing or misplaced atoms or ions in an ionic crystal is called point defects. It is always found in ionic solids. It is of following types-
(1)Stoichoimetric Defects- If defects in crystal are such that the ratio between the cations and anions remains the same as represented by molecular formula, the defects are called stoichiometric defects. It is of following types-
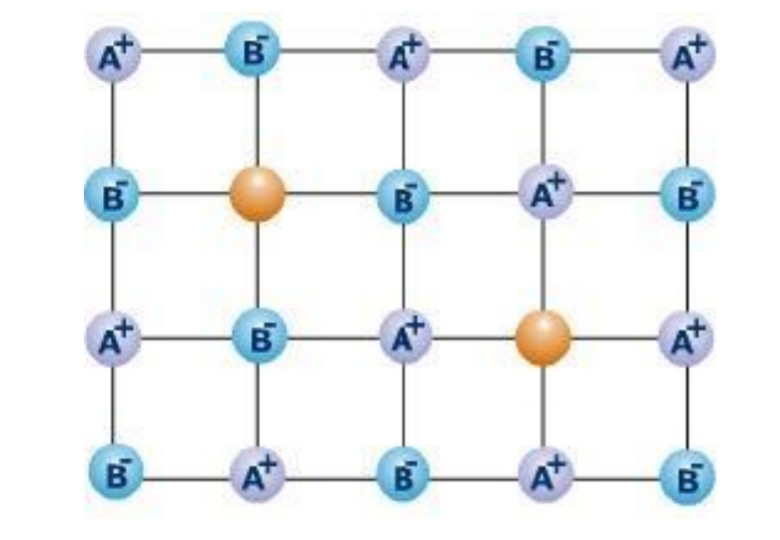
(i)Schottky Defect-If in an ionic crystal of the type A+B- , equal number of cations and anions are missing from their lattice sites so that the electrical neutrality and stoichiometry is maintained , it is called Schottky defect.
This type of defect is shown by those ionic crystals which have-
(i) High co-ordination number
(ii) Small difference in size of cation and anion.
Effect on density-As the number of ions decreases the mass decreases , whereas volume remains the same ,hence density decreases.
Example- NaCl, KCl , CsCl , KBr
Consequences of Schottky defects-
* The compounds having this defect have lower lattice energy and thus lower stability.
*These compound conduct electricity to smaller extant.
*Due to this defect density as well as covalent character decreases.
*It is observed that in NaCl crystal at room temperature there are about 1022 ions 106 Schottky pairs per cm3.This means that there is one Schottky pair defect per 1016 ions.
Frenkel Defect- If an ion is missing from its lattice site and it occupies the interstitial site, electrical neutrality as well as stoichiometry of the compound are maintained. This type of defect is called Frenkel defect.
This type of defect is shown by the crystals which have-
(i) Low co-ordination Number
(ii) Large difference in the size of cations and anions.
Effect on density-Since no ions are missing from the crystal as a whole, therefore density of the solid remains unchanged.
Example- AgCl, AgBr, AgI and ZnS.
Consequences of Frenkel defect-
* The compounds having this defect have lower lattice energy and thus lower stability.
* In Frenkel defect the closeness of similar charges tends to increase the DEC of crystal.
* The small size cation present in interstitial site can move into another site and hence is responsible for electrical conduction.
* AgBr can represent both Schottky and Frenkel defects.
Non stoichiometric Defects-If as a result of the imperfection in crystal the ratios of cations to the anions become different from that indicated by the ideal chemical formula, the defects are called non stoichiometric defects. For example it is very difficult to prepare ferrous oxide with the ideal composition, FeO. What we actually obtain is Fe 0.95 O or FexO , where x = 0.6 to 1.3.
Non stoichiometric compounds are generally formed by transition metals and by lanthanoids and actinides.
Non stoichiometric defect is of following types-
(1) Metal excess defect- This may occur in either of the following two ways-
(i) By anion vacancies- A negative ion may be missing from its lattice site, leaving a hole which is occupied by an electron, thereby maintaining the electrical balance.” When an electron is trapped in the anion vacancies it is called F- centre”.
F- centre is responsible for imparting colour to the crystal.
For example when NaCl is heated in an atmosphere of Na vapors, the excess of Na atoms deposit on the surface of NaCl crystal. Some of the Cl- ions diffuse to the surface where they combine with Na atom which become ionize by losing electrons. These electrons diffuse back into the crystal and occupy the vacant sites created by Cl- ions. These electrons absorb some energy of the white light.
F centre is responsible for imparting yellow colour to NaCl , pink color to the LiCl, and voilet colour to KCl.
This defect is similar to Schottky defect and is found in crystals having Schottky defect.
(ii) By the presence of extra cations in the interstitial sites-Metal excess defect may also be caused by an extra cation occupying the interstitial sites. Electrical neutrality is maintained by an electron present in another interstitial site.
For example when ZnO is heated, it loses oxygen and turns yellow due to following reaction.
Zno ------------ Zn2+ + O2 + 2e-
This defect is similar to Frenkel defect and is found in crystals having Frenkel defects.
• Consequences of metal excess defect-
* Metal excess defect crystals are conductor of electricity due to presence of free electrons.
* They conduct less electricity and called semiconductor. It is due to number of free electrons.
* Crystals are paramagnetic in nature due to this defect.
* Due to this defect crystal acquire colour.
(2)Metal deficiency defect- This defect occurs when the metal show variable valency, i.e. for transition metals. The defect usually occurs due to missing of a cation from its lattice site and the presence of the cation having higher charge (e.g. +2 instead of +1 ) in the adjacent lattice site.
Example- FeO, FeS, and NiO.