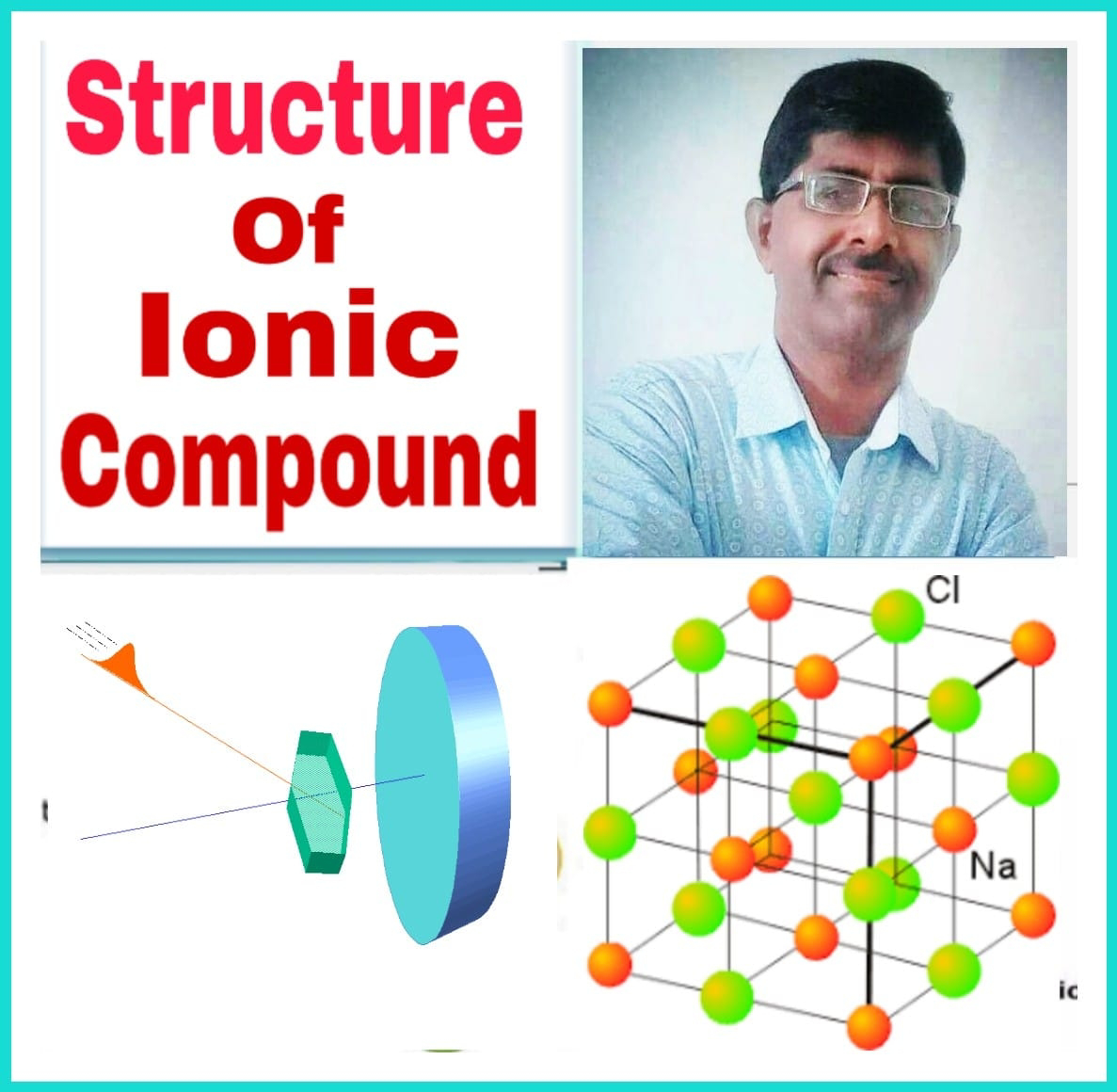
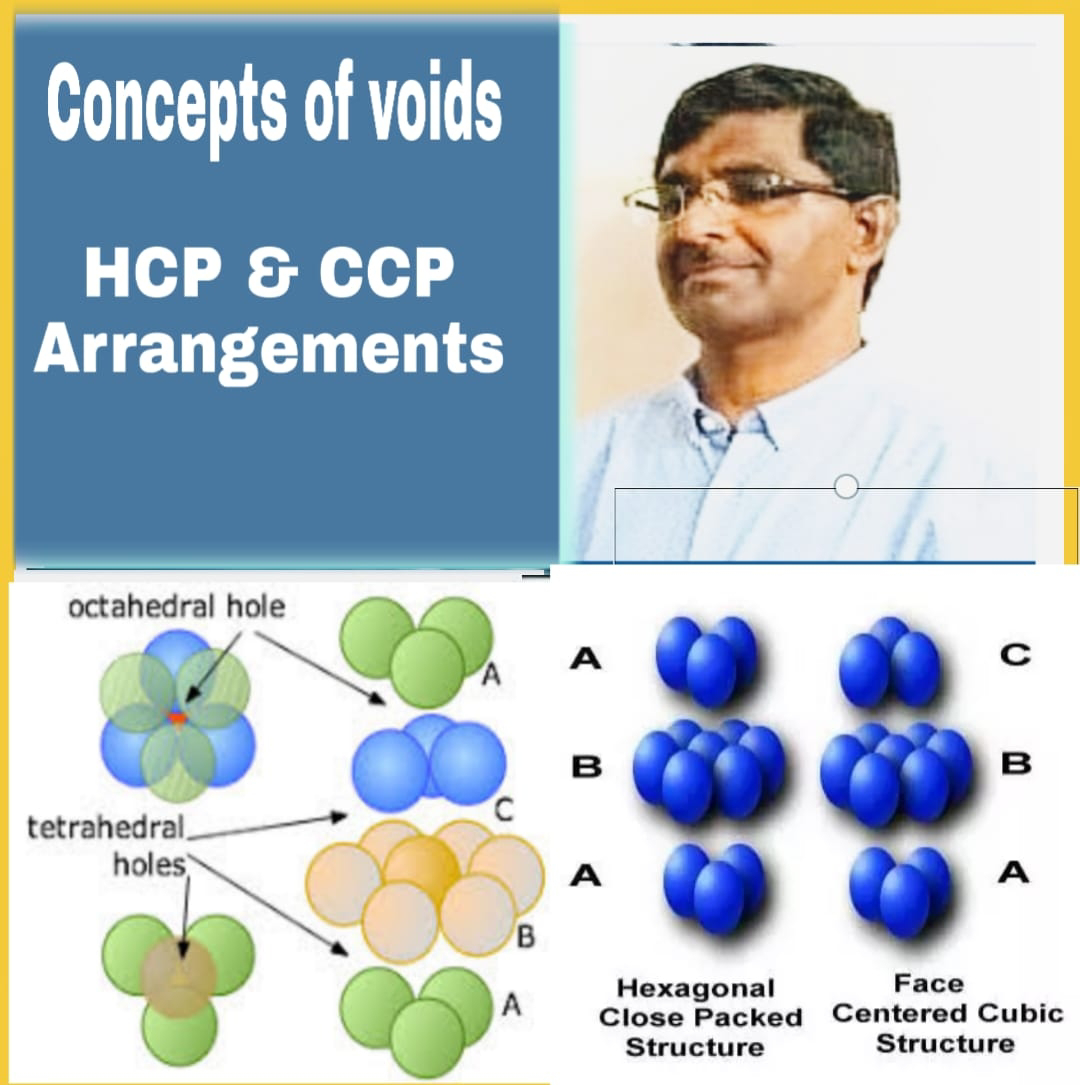
Tertahadral & Octahedral Voids, HCP & CCP
Concepts of Voids, HCP & CCP Arrangements Density of unit cell- Tetrahedral and Octahedral void- Tetrahedral Void- A simple triangular void in a crystal which is surrounded by four spheres is called tetrahedral void. A tetrahedral void is developed when triangular voids have contact with one sphere either in the upper layer or in the lower layer. Tetrahedral void means the tetrahedral arrangement of spheres. . Characteristics of tetrahedral void- (i) Ratio of radii of void and constituent part...