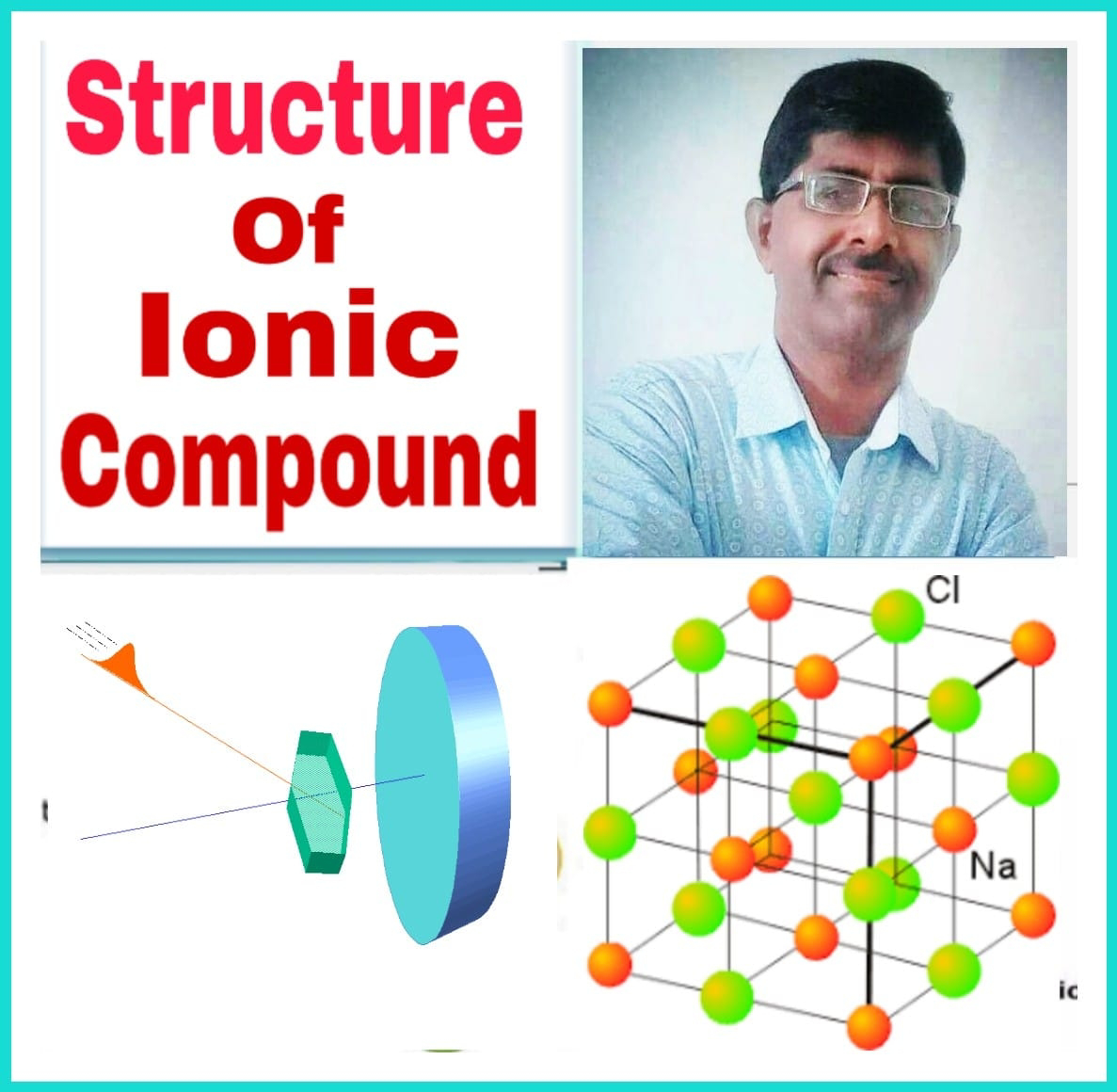
विज्ञान में नई सोच अन्तर्विरोध और विरोधाभासों से ही विकसित होती है

विज्ञान में नई सोच अन्तर्विरोध और विरोधाभासों से ही विकसित होती है हम विरोधाभास ऐसे कथन, ऐसे विचार या ऐसी घटना को कहते हैं जो सामान्य बुद्धि के विरुद्ध होता है। विरोधाभास कई तरह के होते हैं कुछ अन्तर्विरोध के सूचक होते हैं तो कुछ तर्कशास्त्रीय विरोधाभास होते हैं। कुछ भी हो विरोधाभास एक तरह से अन्तर्विरोध ही हैं। विज्ञान का अब तक का इतिहास बतलाता है कि विरोधाभास उस विचार श्रंखला को तोड़ देते हैं जिसका समाज आदी हो जाता है और हमें विवश करते हैं कि हम हर अस्पष्ट और असामान्य बात से मुंह मोड़ लें और बात को गहराई में जाकर देखें। विज्ञान में ऐसे अनेक तर्कशास्त्रीय विरोधाभास होते हैं जो नपेतुले होते हैं, जिनके बारे में यह कहना कठिन होता है कि ये सत्य हैं या असत्य। इस प्रकार के विचित्र तर्कों को वाक्छल या सोफिज्म कहते हैं। एक व्यक्ति ने घोषणा की कि मैँ जो कुछ भी बोलता हूँ, झूठ बोलता हूँ। इसका मतलब है कि उसने इस बार भी झूठ बोला। लेकिन इस विरोधाभास में निष्कर्ष निकलता है कि उसने इस बार उसने खुद के बारे में सच कहा है। लेकिन इस आदमी की बात यदि सच है तो उसने झूठ ही कहा है। इटली म...